- FDA Class I material certification
- Cytotoxicity report passed
- Safe for Mucosal Membrane contact up to 24hrs in duration
- Material can be steam sterilized in an autoclave, or by gamma-ray sterilization
- For more information contact Support@RadTecMD.com
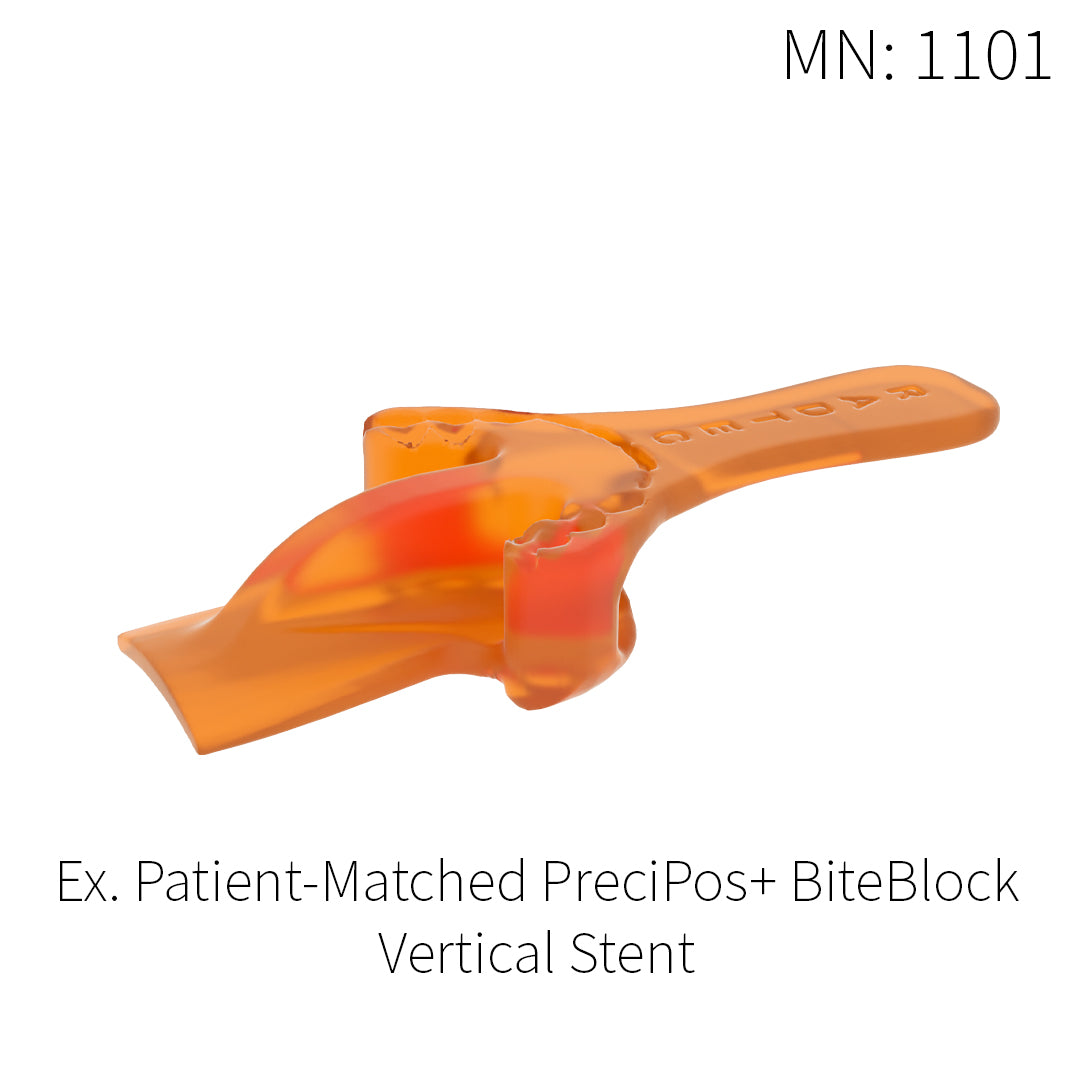
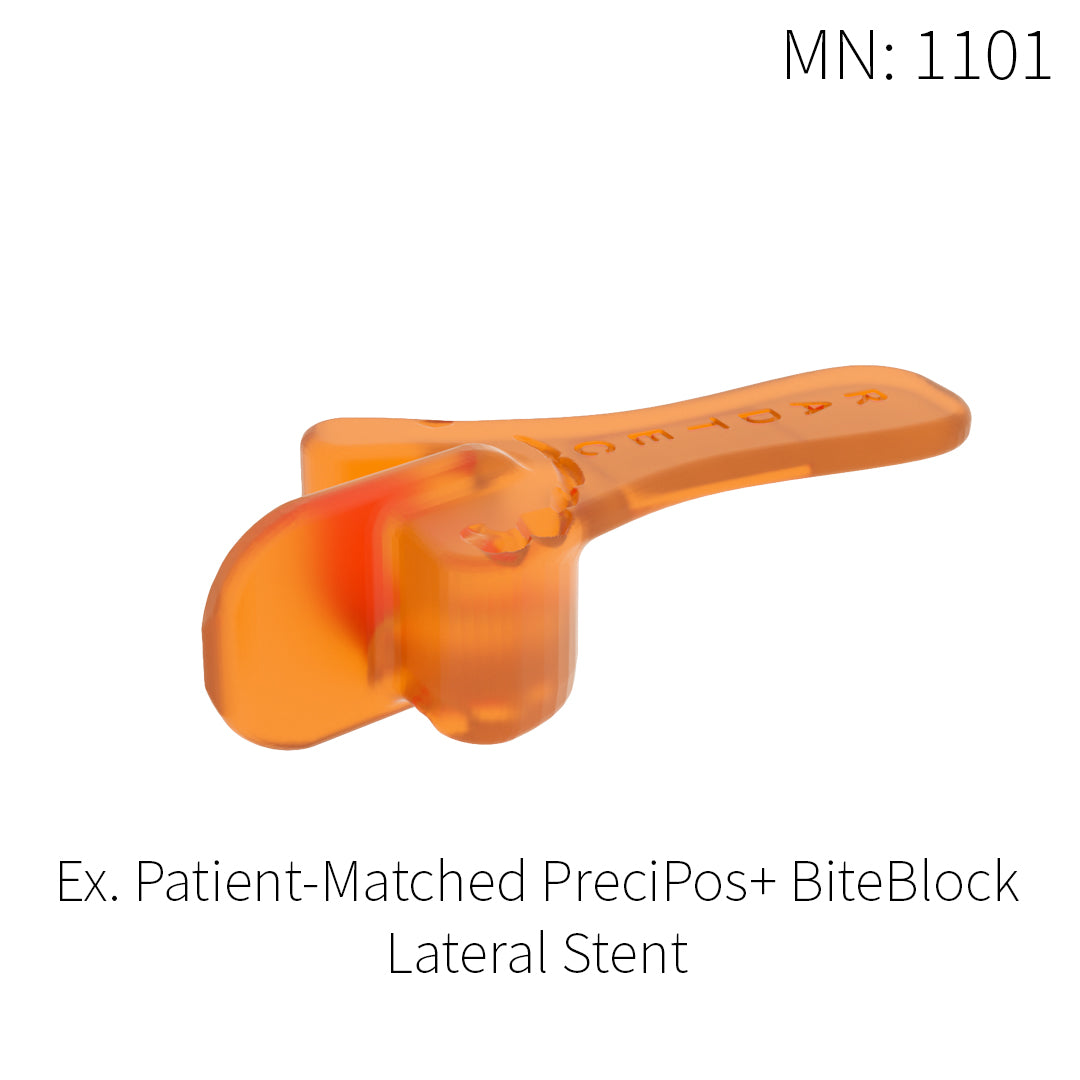
PreciPos+™ BiteBlocks
PreciPos+™ BiteBlocks
Description
With a growing number of patients being treated with Intensity Modulated Radiation Therapy each year, there is a need for ever improving positioning devices. One of the key benefits of IMRT is its precise targeting abilities, allowing the separation of beam entry sites and thus the sparing of healthy tissues.
IMRT treatments for head and neck cancer are often performed five days a week for up to six weeks in duration. Each session involves precise planning and a need for highly accurate positioning. This is where our patient-match PreciPos+™ BiteBlocks come into use. We design a unique device for each IMRT patient to maximize the protection of healthy tissues and structures, while creating a comfortable position that can be replicated every day of the treatment.
Using the latest in 3D design and manufacturing, we create positioning devices that are not only more comfortable than off- the-shelf pieces currently available, they are also more effective. RadTec’s PreciPos+™ BiteBlocks represent a breakthrough in 3D manufacturing that allows patient-matched medical devices to be deployed in everyday use for patients around the world.
Every PreciPos+™ BiteBlock is custom made for the individual, thus maximizing the benefits and comfort of IMRT treatment, while simultaneously improving the quality of life for each patient.
Key Features
Patient-matched intraoral placement devices:
- Designed for the patient based on anatomic structure and treatment plan
- Able to set incisal separation and mandibular position
- Biocompatible, non-toxic materials
- Sterilizable
- Manufactured for the individual with the ability to adapt to most special needs
Specifications
The material has been tested on bio-compatibility according to the ISO 10993-1:2009 at NAMSA, Chasse sur Rhone in France. Device materials meet the applicable requirements of ISO 10993-1 and USP Class VI, and are biologically safe.
- Cytotoxicity test Testreport 177178
- Sanitization Testreport 168884
- Irritation Testreport 168883
- System toxicity Testreport 168888
- Genotoxocity Testreport 170296
The material is tested at NAMSA, Chasse sure Rhone in France, and is certified biocompatible per EN-ISO 109931:2009/AC:2010:
- Non-mutagenic.
- Non-cytotoxic.
- Not induce any erythema or edema reactions.
- Not a sensitizer.
- Not cause systemic toxicity.
Instructions for Use
Instructions for deployment of Open Bite Registration Device:
- Take physical molds of upper and lower teeth using impression putty, or similar materials • It is suggested to use quick set stone when pouring the models to expedite the final check process. It is also suggested that the final check is done when the patient is still in the office, thus saving another visit should something not work properly
- Take the Open Bite stent with the top side up • Denoted with a T on the handle • Make sure the proper incisal opening distance has been chosen
- Place the Open Bite Registration Device in the patients mouth and have them open and close their bite until a natural, comfortable position has been found for the positioning of the mandible
- Using blue mouse or other injectable molding material, inject mouse along the top and bottom of each arm • pushing from front to back while applying slight pressure to allow mouse to work between the teeth and registration arms
- Allow mouse to cure as recommended by manufacturer
- Once mouse is set have patient open their bite and remove the Open Bite Registration Device
- Place the upper and lower molds onto the Open Bite Registration Device and insure a proper fit was achieved
- Once complete determine what method will be used to transfer device and molds to: RadTec Medical Devices Inc. 170 Glenn Way Suite #5 San Carlos, CA 94070
Instructions for Deployment of PreciPos+ BiteBlocks:
Inspect for cracks or other defects. If defects are found, discontinue use.
- If desired, the device may be sterilized prior to use. See validated sterilization parameters below.
- Once inspected, place the device in the subject’s mouth, fitting both upper and lower teeth into the appropriate positions.
- Make sure the tongue is positioned, as needed, and inspect fitment.
- If needed, prepare a thermoplastic mask, and form it onto the patient with the Precipos+ BiteBlock in place. Fitment of the positioning mask should leave enough room without putting pressure on the handle.
- Have the patient verify that the position is comfortable, and they can maintain this position during treatment.
- Once treatment session is complete, remove mask and bite block
Tips for ease of use:
- Instruct the patient to breathe through their nose, not mouth.
- Instruct the patient to minimize swallowing with Precipos+ BiteBlock in place (suction may be applied if available).
STERILIZATION INSTRUCTIONS
Sterilization:
The Precipos+ BiteBlock is supplied non-sterile and may be sterilized prior to use. The device should be packaged in an FDA-cleared pouch or wrap designed for autoclaving at the selected cycle and designed to maintain sterility after processing. The recommended sterilization method and parameters are listed in the table below.