Lollipop BiteBlocks™
Lollipop BiteBlocks™
Description
Lollipop Bite Blocks™ are an “off the shelf” intraoral positioning system that can be used without any customization. Designed to serve patients on a constricted time line or that are unable to use a custom IPD for whatever the reason may be. Each stent incorporates design characteristics that we learned through the creation of our custom IPD’s and are designed to fit specific positioning needs. If you would like more information, please contact us and we would be more than happy to answer any questions you may have.
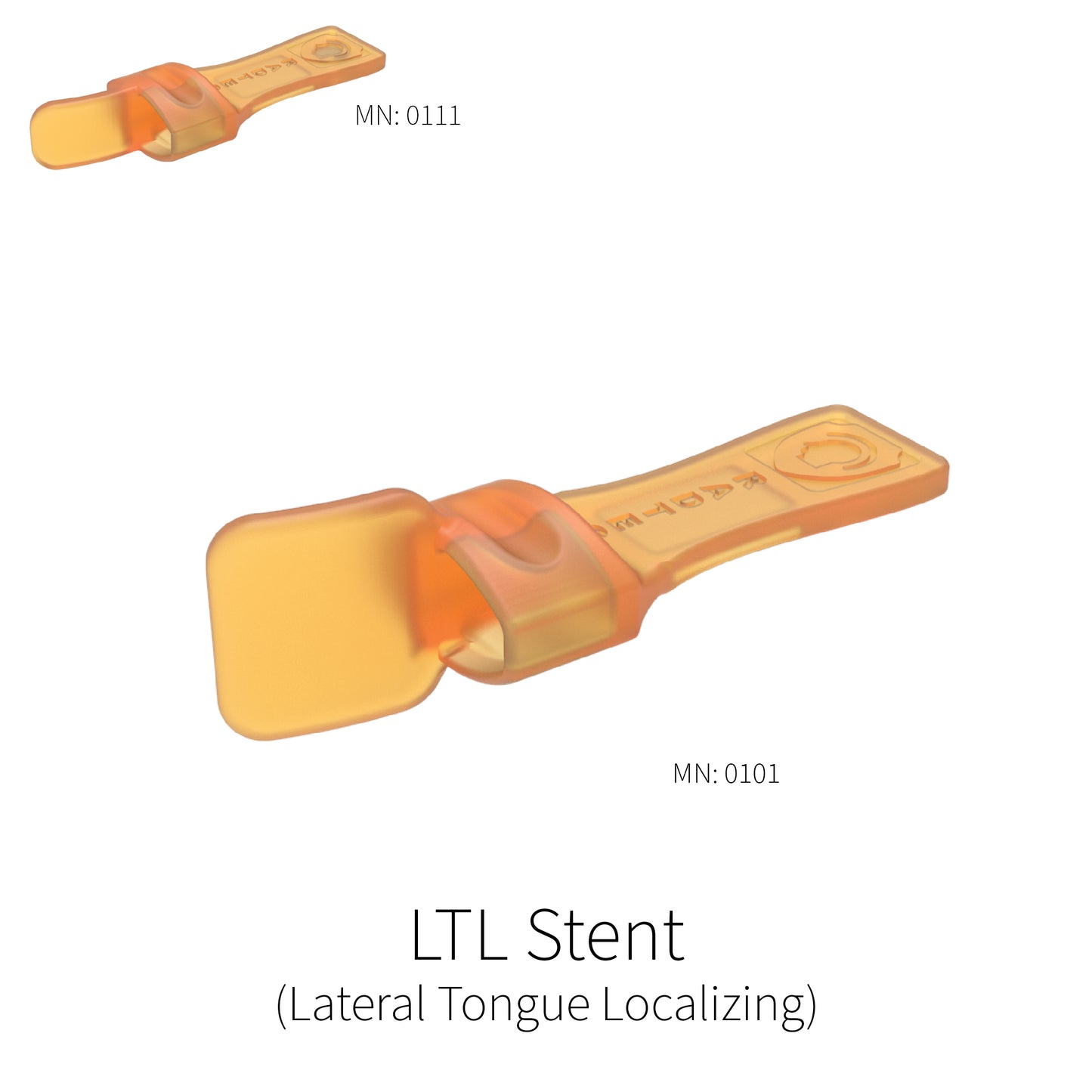
Lateral Tongue Localizing (L.T.L) Stent
This stent positions the patients tongue either to the right or left in the patient’s mouth to help move the tongue away from the field of treatment. It is intended for treatment sites that may be more hemispherically located, for instance on the outer portion of the cheek or jaw where having the tongue pushed to the opposite side of the mouth creates a large enough separation that the effective dose of radiation is drastically reduced.
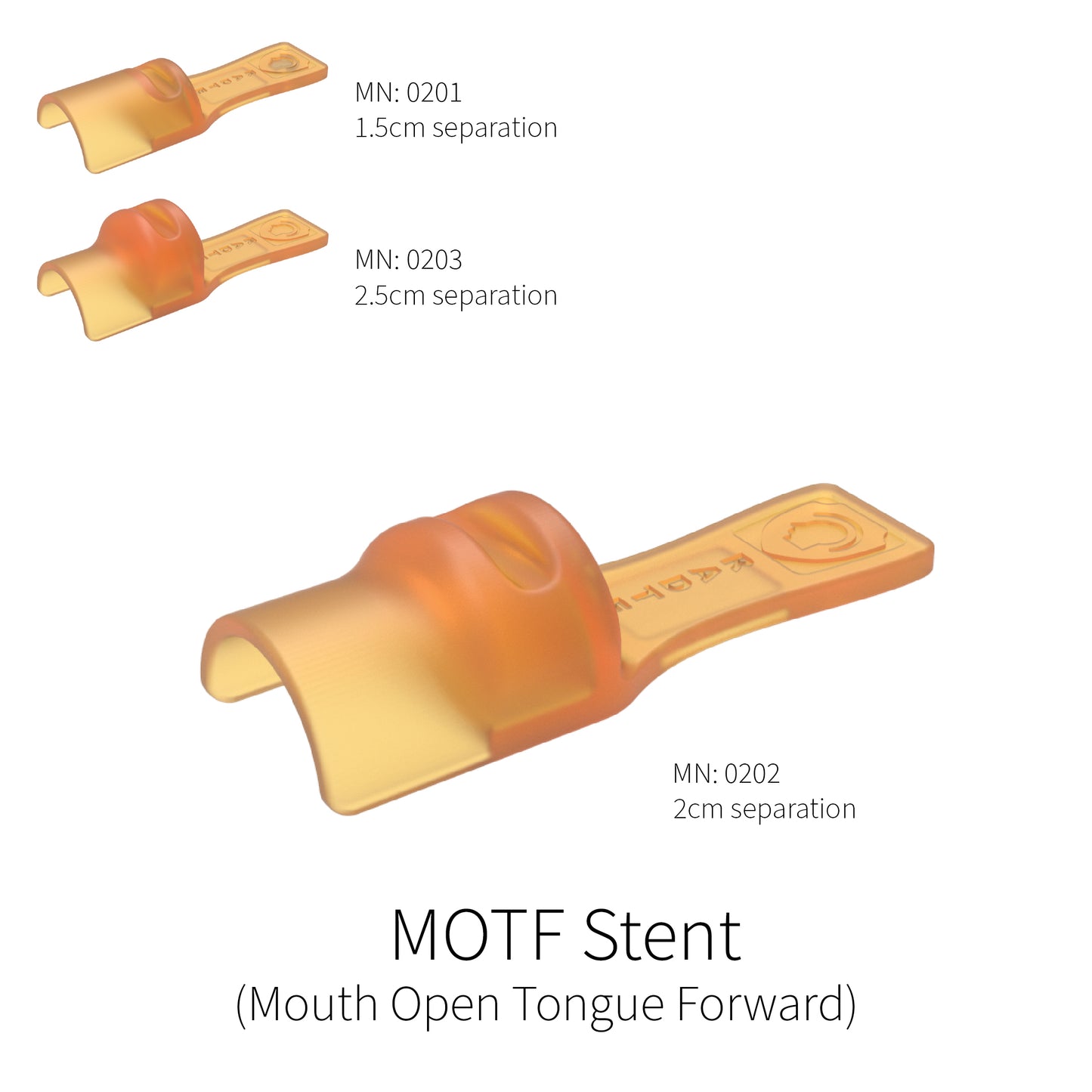
Mouth Open Tongue Forward (M.O.T.F) Stent
This stent positions the jaws with a wide incisal separation and has a tongue positioning paddle that not only covers the top portion of the tongue, it also opens an area over the lower front teeth to allow the tongue to be positioned as far forward as possible. These stents are intended for base of tongue, esophageal, and neck lymph node treatment sites as well as anywhere that elongating the tongue and creating separation of the jaws is desired.
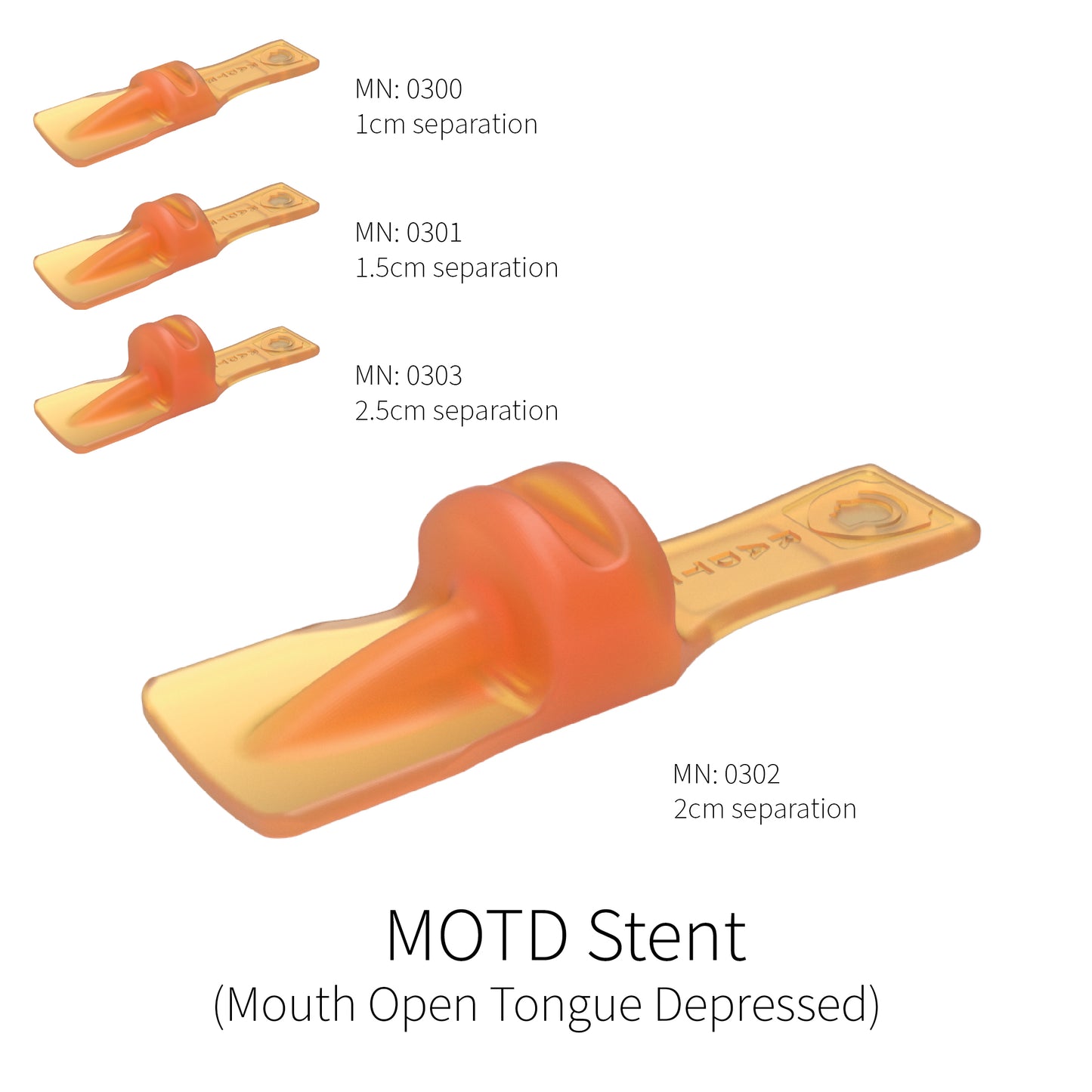
Mouth Open Tongue Depressed (M.O.T.D.) Stent
This stent has a range of incisal separations that create separation between the upper and lower jaw while the tongue positioning paddle is flat against the lower teeth. This stent allows for the tongue to be kept low against the base of the mouth with a large separation between the upper and lower jaw. These are most useful for treatment sites in the maxilla, the upper palate, and the nasopharyngeal regions.
Key Features
Customized intraoral placement devices:
- Designed for the patient based on anatomic structure and treatment plan
- Able to set incisal separation and mandibular position
- Biocompatible, non-toxic materials
- Sterilizable
- Manufactured for the individual with the ability to adapt to most special needs
Instructions for Use
The Lollipop Bite Block™ is to be used only under the care and supervision of trained medical personnel. Intended for single patient use, with multiple sessions not to exceed a maximum of 24hours total time in mucosal membrane contact. See materials specifications for more information about specific material designations.
INSTRUCTIONS FOR DEPLOYMENT OF LOLLIPOP BITEBLOCKS
Inspect for any flaws or defects prior to use.
- If desired, the device may be sterilized prior to use. See validated sterilization parameters below.
- Once inspected, place the device in the subject’s mouth, fitting both upper and lower teeth into the appropriate positions.
- Make sure the tongue is positioned, as needed, and inspect fitment.
- If needed, prepare a thermoplastic mask and form it onto the patient with the Lollipop BiteBlock in place. Fitment of the positioning mask should leave enough room without putting pressure on the handle.
- Have the patient verify that the position is comfortable, and they can maintain this position during treatment.
- Once treatment session is complete, remove mask and biteblock
Tips for ease of use:
– Instruct the patient to breathe through their nose, not mouth.
– Instruct the patient to minimize swallowing with Lollipop
BiteBlock in place (suction may be applied if available).
STERILIZATION INSTRUCTIONS
The Lollipop Biteblocks are supplied non-sterile and may be sterilized prior to use. The recommended sterilization method and parameters are listed in the table below. The device should be packaged in FDA-cleared pouch or wrap designed for autoclaving at the selected cycle and designed to maintain sterility after processing. The recommended sterilization method and parameters are listed in the table below.
* This device can be steam sterilized in an autoclave, or by gamma-ray sterilization
*NOT INTENDED FOR RESALE BY ANY THIRD PARTIES